This quiz is for registered users only.

Urological Disorders
II. ABSTRACT
Lower urinary tract symptoms (LUTS) from genitourinary conditions such as overactive bladder, detrusor-sphincter dyssynergia, interstitial cystitis, benign prostatic hyperplasia, and prostatitis are relatively common. Currently available medical and surgical management for these conditions leaves room for improvement. Beyond applications in plastic surgery, dermatology, and neurology, botulinum neurotoxin (BoNT) injection has been studied for the management of LUTS caused by these genitourinary conditions. BoNT affects hypercontractability, hypersensitivity, and glandular hypertrophy. Dose and duration vary according to the disease being treated as well as the particular BoNT product. In general, over the past 6 years, numerous urological studies have shown promising results with symptom relief of up to 6 months or longer as well as improvement in quality of life. BoNT is generally well tolerated, with some impact on post-void residual urine and urinary tract infection. The optimal dose dilution, number, and location of injection are still under investigation. Botulinum neurotoxin shows promise for treating a variety of genitourinary conditions affecting the lower urinary tract.
III. UROLOGICAL DISORDERS OVERVIEW
Lower urinary tract symptoms (LUTS) result from a range of disorders of the genitourinary system, including (Figure 1)1:
- Detrusor-sphincter dyssynergia (DSD)
- Overactive bladder (OAB) due to neurogenic detrusor overactivity (NDO)
- OAB due to non-neurogenic (idiopathic) detrusor overactivity (IDO)
- Interstitial cystitis (IC)
- Benign prostatic hyperplasia (BPH)
- Prostatitis
- Storage (such as urgency, nocturia, and frequency)
- Voiding (such as reduced stream, intermittency, and hesitancy)
- Post-micturition (such as terminal dribble and feeling of incomplete emptying)
LUTS is a very common symptom complaint among both men and women. Although a great deal of emphasis has been placed on the occurrence of LUTS in men, the prevalence of LUTS does not differ by gender or race; 72.3% of men and 76.3% of women experience at least one symptom occasionally. However, treatment seeking for LUTS is low,2 so the true prevalence may be difficult to ascertain. In general, voiding symptoms are more common in men, while storage symptoms predominate in women.2
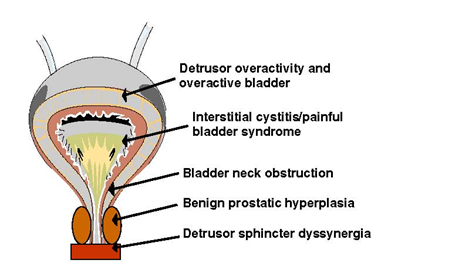
Figure 1. Sites along the genitourinary tract implicated in lower urinary tract symptoms. Adapted with permission from Chancellor.1
The lower urinary tract (LUT) encompasses the urinary bladder and the urethra and has two main functions: storage and voluntary voiding of urine. As described for the individual conditions below, LUTS can result from dysfunctions at various locations of the lower urinary tract functional unit as well as at the level of the prostate.2 During bladder filling, the internal sphincter remains contracted because of tonic background adrenergic tone. The detrusor muscle produces coordinated bladder contractions, leading to bladder voiding. This muscle is innervated by the pudendal nerve; cholinergic fibers predominate in the body of the bladder, but adrenergic fibers are present in the body and the neck of the bladder. The LUT is innervated by three groups of peripheral nerves: sacral parasympathetic, lumbar sympathetic, and sacral somatic nerves.
The bladder is surrounded by the detrusor muscle bundles, whereas the urethra contains a dual sphincter mechanism: the internal sphincter is a smooth muscle that surrounds the vesical neck and the posterior urethra and receives adrenergic and cholinergic innervation; the external sphincter is composed of two parts: a smooth component that is intrinsic to the urethra and is mainly under autonomic control as well as a striated component that encircles the membranous urethra and is innervated by the pudendal nerve (Figure 2).4
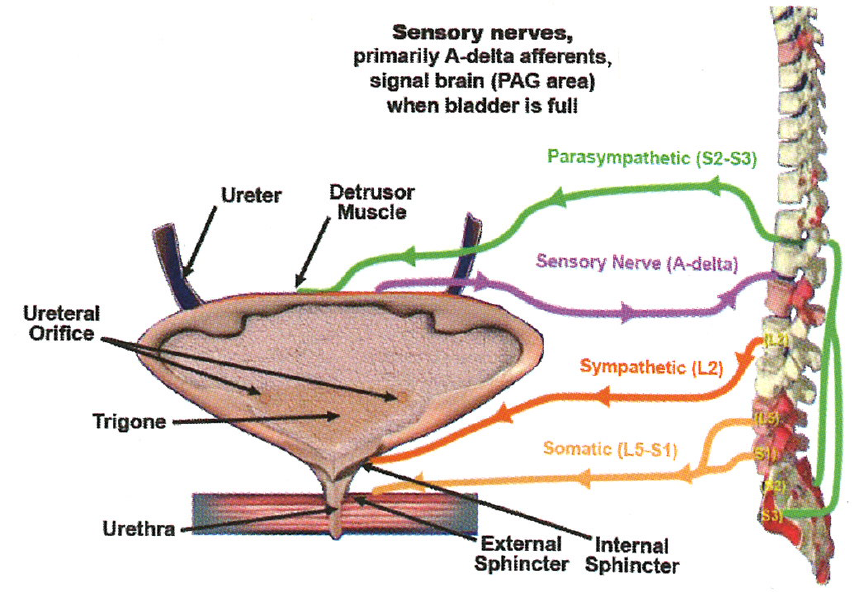 PAG = periaqueductal gray.
PAG = periaqueductal gray.
Figure 2. Lower urinary tract anatomy and innervation. Adapted with permission from Chancellor. 4
Urine is stored when the external urethral sphincter muscle (somatic) and the internal urethral sphincter muscle (sympathetic) are contracted and the detrusor muscle and sacral parasympathetic activity are inhibited through sympathetic mediation. During the act of micturition, descending pathways originating from the pontine micturition center inhibit the external urethral sphincter and sympathetic outflow (inhibition of the vesicosympathetic reflex), activate parasympathetic outflow to the bladder, and activate parasympathetic outflow to the urethra. Neural pathways controlling LUT function are organized as simple on-off switching circuits that control urinary bladder filling and emptying. Alpha and C afferent pathways initiate micturition.4 Alpha fibers exhibit graded response to passive distention, whereas C fibers, activated by inflammation and noxious stimuli, have a much higher threshold. Bladder fullness is detected by receptors in the bladder wall that activate the sacral parasympathetic nerves; the impulses reach the cerebral cortex through the spinothalamic tract.
Since such a wide array of conditions may be associated with LUTS, including urinary tract infections (UTIs), symptoms alone cannot be used to make a definitive diagnosis. However, signs suggestive of LUT dysfunction are available; these include leakage on coughing as well as observations from frequency volume charts, pad charts, and quality-of-life (QoL) questionnaires. Urodynamic observations are also important. Signs, symptoms, and urodynamic observations, along with evidence or exclusion of a nonurodynamic process, are relevant to the diagnosis.3
IV. DETRUSOR-SPHINCTER DYSSYNERGIA (DSD)
Detrusor-sphincter dyssynergia (DSD) is a spastic condition commonly seen in patients with neurologic insults, including spinal cord injury (SCI) and multiple sclerosis (MS). DSD affects the nerves that control the skeletal and smooth muscles of the urinary system, causing a lack of coordination between the bladder and the external sphincter.
It is difficult to determine the overall epidemiology of DSD, but some small studies are available investigating prevalence in specific risk groups. In one Turkish study of 51 patients with post-traumatic SCI, 29 of the 36 patients (80.5%) with suprasacral injury had DSD, while 2 of the 14 patients with sacral injuries (14.3%) developed DSD.5 In another study conducted in 82 patients admitted for stroke, 22 had incomplete bladder emptying, and 50% of those had DSD. Among these stroke patients, development of DSD was associated with a longer onset to evaluation interval and spasticity of the stroke-affected lower limb.6 A Turkish study examined etiologies of voiding dysfunction among 249 MS patients referred to a urodynamic unit. Among these patients, 75 had urodynamic assessment, and 30 (40%) of these had detrusor overactivity with DSD.7
DSD results in poor bladder emptying and high intravesical pressure, which may lead to autonomic dysreflexia; serious urinary tract infection; development of upper tract dysfunction associated with ureteral reflux from high pressures; resulting kidney dysfunction; and, potentially, premature death. The underlying cause of the disorder seems to affect the clinical course and features. For example, MS patients with DSD rarely progress to upper tract deterioration, which is more common in patients with SCI.7
In DSD, lesion(s) between the sacral and pontine micturition centers can cause loss of descending input, which leads to insufficient relaxation as well as uncoordinated contraction of the vesical sphincter during detrusor contraction. The result is that the external sphincter remains in a contracted state, leading to outlet obstruction as the bladder attempts to expel urine.8
Currently, there is no consensus regarding diagnosis of DSD in terms of specifics of electromyographic or voiding cystourethrographic analyses, although there is no evidence that the combination of these modalities is beneficial.9 Patients are often evaluated with medical history and physical examination, post-void residual urine volumes, culture of urine samples, electromyography, mechanical and electrical stimulation measurement of bulbosphincteric reflexes, determination of urethral pressure profiles, excretory urography, voiding cystourethrography, and manual muscle tests.10
As reviewed by Schurch and colleagues, the current treatments for DSD leave room for improvement. Anticholinergics are used to block the efferent parasympathetic innervations in DSD, but these medications are associated with troublesome side effects and may be insufficient to restore continence. Sacral root rhizotomy has a role but should be restricted to patients with complete suprasacral cord lesions; this cannot be used in men who want to preserve reflex erections. Phenol injections into the subtrigonal region of the bladder usually provide only a transient benefit. Capsaicin is effective in patients with MS or spinal cord injury (SCI); however, the data are mixed, and some patients are nonresponders. The diterpene analog resiniferatoxin is more potent than capsaicin and may have some benefit. Stimulation of the pudental nerve may provide some short-term benefit, but long-term benefits are rare.11 Sphincterectomy is a surgical option,10 but most urologists agree that medical management options should be exhausted before resorting to this procedure.
Animal studies have demonstrated that botulinum neurotoxin (BoNT) injected into rat bladder and urethra markedly inhibited the release of labeled norepinephrine and acetylcholine.12,13 Blocking norepinephrine release may inhibit sympathetic transmission and smooth muscle dyssynergia. Only two of the seven known BoNT serotypes are used clinically: BoNT-A and BoNT-B. In DSD patients, percutaneous or cystoscopic injection of the sphincter with BoNT-A (onabotulinumtoxinA) has been shown to reduce urethral pressure and post-void residual (PVR) urine volume, with an average effect duration of 50 to 60 days.10,14 Typically, onabotulinumtoxinA treatment of DSD produces clinical improvement within 5 to 7 days, eliminating the need for surgical treatment.15
An early investigation of onabotulinumtoxinA injection in patients with SCI and DSD reported efficacy in 21 of 24 patients, with transurethral injections having a greater effect than transperineal injections on maximum urethral pressure.16 One randomized, controlled trial compared the efficacy and tolerability of onabotulinumtoxinA (100 U) and lidocaine (4 mL of 0.5%) applied in the external urethral sphincter with a single transperineal injection in patients with SCI, DSD, and chronic urinary retention.17 PVR volume (assessed three times daily on days 1, 7, and 30 after each injection) decreased, and clinical symptoms improved significantly more in patients treated with onabotulinumtoxinA than in those treated with lidocaine.17
In a study including 29 DSD patients who received urethral injection of onabotulinumtoxinA, 28% subjectively reported an excellent result and 52% reported significant improvement.18 Smith et al reviewed the 6-year experience of 110 patients with voiding dysfunction resulting from a variety of LUT disorders, including DSD, who received onabotulinumtoxinA injections into the bladder or urethra.19 Maximal efficacy of onabotulinumtoxinA occurred between 7 and 30 days post-injection, with neither systemic nor local complications observed. Over 67% of the 110 patients reported a decrease or absence of incontinence, with a decrease in both daytime and nighttime voiding symptoms, as well as improved QoL symptom scores.19 Other BoNT-A products as well as the BoNT-B product rimabotulinumtoxinB are also used clinically to manage DSD,20 but the evidence base on these agents is less established.
Although the results of studies of BoNT in DSD are encouraging, they are difficult to interpret because of the small numbers of patients, wide range of doses, and variable outcome measures employed in these studies. The 2004 International Consultation on Incontinence (ICI) considered there to be fair, research-based evidence (individual cohort studies, including randomized, controlled studies) to support the recommendation of BoNT for DSD. ICI recommendations for practice stated that BoNT sphincteric injections may be an alternative to sphincterotomy for DSD (personal communication, Michael B. Chancellor, MD). Controlled studies with well-defined patient populations and validated and reproducible outcome measures are needed.
V. OVERACTIVE BLADDER AND DETRUSOR OVERACTIVITY
Overactive bladder (OAB) syndrome is characterized by urinary frequency and urgency, with or without urgency incontinence.3,21 The underlying cause of OAB syndrome is detrusor overactivity (urodynamically demonstrable involuntary bladder contractions), which is subdivided into neurogenic detrusor overactivity (NDO) or idiopathic (non-neurogenic) detrusor overactivity (IDO).3OAB syndrome can have a significant impact on the patient’s QoL, affecting interpersonal and sexual relationships and causing the patient to feel embarrassment and loss of self-confidence.
OAB is a very common disorder. In the United States, OAB is estimated to occur in 16% of men and 16.9% of women.22,23 A recent study conducted in Europe found a prevalence rate closer to 12%; with approximately 50% of those with OAB experiencing significant bother.24 Age is a risk factor. A Japanese epidemiological survey, which reported a 12.4% incidence of OAB in the general population over age 40, found that prevalence rates increase with age, and that 37% of those older than age 80 have OAB.25
As mentioned previously, overactive bladder (OAB) syndrome is characterized by urinary frequency and urgency, with or without urgency incontinence. Of those patients with OAB, one third are troubled by urgency incontinence (OAB “wet”) and two thirds are not (OAB “dry”).22 The clinical course of OAB is variable. In one study from Australia that evaluated longitudinal outcomes in 1975 women with a sole urodynamic diagnosis of IDO, the median duration of symptoms was 13 years (range, 9–18 years). Improvement was achieved in 35% of women with treatment. Disease symptoms fluctuated in severity, and QoL was worse in nonresponders (P<.0001). Urge incontinence and nocturia were associated with treatment failure (P<.001 and P=.04, respectively).26
Proposed factors involved with overactive bladder include neurogenic and/or myogenic causes. Potential neurogenic factors include damage to the central neurologic pathways, resulting in loss of peripheral inhibition, reduced suprapontine inhibition, injury to axons within the spinal cord, increased LUT afferent output, and enhancement of excitatory neurotransmission within the micturition reflex pathways.27
OAB is usually diagnosed by measurement of symptoms—urinary urgency (sensation of incomplete emptying of the bladder), frequency of at least 8 times in 24 hours, and nocturia, with or without incontinence for more than 6 months in duration.28 For a differential diagnosis, it is important to look at urinalysis, urine cytology, urine culture, and cervical ureaplasma culture. Further, an abdominal or pelvic examination is needed to make sure the lower abdomen, pelvic floor, and bladder are not tender, which might be indicative of another process.27
Antimuscarinics are the first choice of agents in the pharmacotherapy of OAB and are supported by level I evidence (ie, evidence obtained from at least one properly designed randomized clinical trial). These agents have demonstrated acceptable efficacy, safety, and improvements in QOL.21 However, because muscarinic receptors are also found in the salivary glands, gastrointestinal smooth muscle, eyes, heart, and brain, antimuscarinics can have side effects such as dry mouth, constipation, blurred vision, tachycardia, and cognitive problems.21,29 For the patient who is antimuscarinic refractory, sacral neuromodulation and surgical augmentation cystoplasty can be considered.
Nonpharmacological approaches that have been studied for LUTS include pelvic floor training, biofeedback, and behavioral modification, in which there is an analysis of the alteration of the relationship between the patient’s symptoms and his or her environment as a means for treating maladaptive voiding patterns.3 Furthermore, some patients may benefit from behavioral training and toileting assistance to expand the intervals between voiding and get on a scheduled toileting routine.28
In treating patients with urgency incontinence due to NDO, a dose of 300 U of onabotulinumtoxinA has been commonly selected for injection into the detrusor, and excellent results have been achieved.16,30-33 Although previous studies have shown that a 200-Unit onabotulinumtoxinA injection had a similar therapeutic result and effect on the QoL index as 300 U, the dose of 200 U might not be adequate for patients who wish to become completely dry.16,34,35 OnabotulinumtoxinA treatment of NDO owing to SCI has been reported to provide satisfactory results lasting up to 9 months.31,32 Seventy-three percent of patients with neurogenic bladder resumed a continent condition after treatment.32
Herschorn and colleagues36 presented a randomized, double-blind trial of onabotulinumtoxinA injection for treatment of NDO and urinary incontinence at the International Continence Society (ICS) 2009 meeting. In this study, 57 subjects with NDO because of either SCI or MS received a single intravesical injection of 300 U onabotulinumtoxinA or placebo and were followed up to 36 weeks. The investigators found that urinary incontinence episodes decreased at Week 6 (-57.1% change from baseline, P<.001), with persistence up to Week 36 in the onabotulinumtoxinA arm. Similar adverse events were seen in both arms, and patients reported an improvement in QoL.
Other BoNT products have been studied for OAB as well. An alternative BoNT-A product, abobotulinumtoxinA, has been studied at 2 different doses (500 or 1000 IU) endoscopically injected into the detrusor muscle in 22 patients with drug-resistant NDO. No significant differences were found between the efficacy duration of the two doses (7.7 months with 500 IU vs 8.5 months with 1000 IU). Maximum cystometric capacity, reflex volume, bladder compliance, and patient satisfaction improved after each treatment. The authors suggest that the clinical efficacy of 750 IU of abobotulinumtoxinA should be studied in an effort to maximize efficacy and avoid unnecessary complications.37 Tachyphylaxis can occur because of the development of antibodies with BoNT products. In a study examining anti-abobotulinumtoxinA antibody formation, 44 children with neuropathic bladder were enrolled and classified into groups without prior injection (Group 1), with recent prior injections (Group 2), and with injections 3 to 36 months prior to study. Healthy controls were included. Antibody levels increased 1 to 2 months after the last injection in 3 (38%) of group 1 and 5 (71%) of group 2. The antibody level in group 2 was not higher than controls, and all patients had complete or partial improvement in incontinence score except for 1 patient from group 1. The authors conclude that the increased antibody formation is not permanent and may not be associated with treatment failure.38
RimabotulinumtoxinB (BoNT-B) was studied in a dose-escalation study of in 15 women with OAB. In this study, rimabotulinumtoxinB was dosed from 2500 U to 15,000 U, with an average duration of 43 days with 5000 U. All but 1 patient responded, and the degree of response was similar across all doses.39 Ghei and colleagues conducted a randomized, double-blind, placebo-controlled trial in 20 patients with detrusor overactivity (either neurogenic or non-neurogenic) unresponsive to oral antimuscarinic agents. There were clinically significant differences favoring average voided volume, urinary frequency, and episodes of incontinence between the rimabotulinumtoxinB and placebo-treated patients.39 However, the authors note that this therapy only has an effect for 6 weeks, while BoNT-A confers a benefit of 24 weeks.40 Therefore, based on this study as well as a more recent study showing that beneficial effects of rimabotulinumtoxinB wear off by 10 weeks,41 investigators suggest that rimabotulinumtoxinB may have a role mostly in patients who have experienced tachyphylaxis to BoNT-A but is not appropriate for widespread use.40,41
The 2004 International Consultation on Incontinence (ICI) considered there to be fair, research-based evidence (individual cohort studies, including randomized, controlled studies) to support the recommendation of BoNT for OAB. ICI recommendations for practice stated that BoNT injections may provide an alternative therapy for detrusor overactivity if conventional therapy fails (personal communication, Michael B. Chancellor, MD).
Optimal Delivery
In terms of dose optimization with BoNT-A, in previous studies using onabotulinumtoxinA for OAB or IDO, most investigators used detrusor injections of 200 U or 300 U of onabotulinumtoxinA.42 Kessler et al treated 11 patients with IDO with detrusor injections of 300 U of onabotulinumtoxinA,42 and the maximal bladder capacity increased from 220 mL to 340 mL. However, 9 patients needed clean intermittent catheterization (CIC) due to large PVR volume. Rajkumar et al treated 15 women with IDO with detrusor injections of 300 U of onabotulinumtoxinA, and 14 had improvements in urgency and frequency. The therapeutic effects lasted for 5 to 6 months.43 Popat et al used 200 U of onabotulinumtoxinA for 31 IDO patients. Although significant improvement in bladder capacity was noted after treatment, 19% of the patients needed CIC post-treatment.44 Schulte-Baukloh et al used 300 U of onabotulinumtoxinA detrusor and urethral injections for 7 women with OAB without detrusor overactivity. The bladder capacity increased by 20%, and all patients could void without the need for CIC.45 In a study by Kuo, detrusor injections of 200 U of onabotulinumtoxinA provided a 73.3% success rate in 30 IDO patients, with a mean therapeutic duration of 5.3 months.46 A recent meta-analysis conducted by Anger and colleagues47 summarized 23 full articles of intravesical onabotulinumtoxinA injections in idiopathic OAB, including three randomized, placebo-controlled trials, accounting for 99 patients. Treated patients had 3.88 fewer incontinence episodes per day. While QoL was also improved, onabotulinumtoxinA-treated patients had a 9-fold higher increased risk of elevated PVR volume compared with placebo-treated patients.
Further study using suburothelial injections of onabotulinumtoxinA at a dose of 200 U revealed therapeutic results (85% success rate) equivalent to those achieved with 300 U of onabotulinumtoxinA in other studies.48 Flynn and colleagues studied onabotulinumtoxinA dosed at either 200 U or 300 U administered into the detrusor muscle for 22 patients with idiopathic OAB syndrome. The active groups showed improvements in daily incontinence scores, pads changed per day, and QoL. However, 26% had a PVR volume of 200 cm or greater, and 1 subject required intermittent catheterization.49 In another recent IDO study comparing 200 U, 150 U, and 100 U of onabotulinumtoxinA, Kuo found that 100 U achieved excellent results at 3 months (achieved in 35% of patients) but lower doses had higher failure rates in NDO.34 Recently, the dose of onabotulinumtoxinA for OAB or IDO was further reduced to 100 U by many investigators, and a satisfactory outcome was still achieved. Werner et al treated 26 women with IDO, with a 53% success rate.50 Schmid et al treated 100 IDO patients, with an 88% success rate.51 Cohen found similar success rates with 100 U vs 150 U of onabotulinumtoxinA in a small study in idiopathic OAB syndrome, but noticed a trend toward better outcomes for OAB-wet type with the 150 U vs the 100 U dose.52
Dmochowski et al evaluated onabotulinumtoxinA over a range of doses, from 50 U to 300 U, in a phase 2, multicenter, randomized, double-blind study of 313 patients with idiopathic overactive bladder and urinary urgency incontinence.48 In this study, doses above 150 U of onabotulinumtoxinA did not appear to add much incremental benefit, particularly when balanced with PVR urine-related safety parameters. Data from this trial suggests that a dose of 100 U may be the dose that appropriately balances the symptom benefits with the post-void residual urine volume related safety profile.
From a cost perspective, treatment with intradetrusor onabotulinumtoxinA has an advantage compared with sacral neuromodulation and surgical augmentation cystoplasty for patients with OAB that is antimuscarinic refractory. In an analysis of initial and follow-up costs based on claims analysis over the course of a 3-year period, the cumulative cost associated with sacral neuromodulation was $25,384 to $27,357; for BoNT treatment, $4,586 to 11,476; and for augmentation cystoplasty, $12,315 to $16,830. Such economic differences should be considered along with data on outcomes and risks in analyzing these therapies as options for antimuscarinic treatment failures.53
Other Emerging Treatment Options
Currently, alternative medical therapies for OAB with fewer side effects than antimuscarinic agents are being investigated in randomized clinical trials. These include21:
- aprepitant, an NK-1–receptor antagonist used to treat chemotherapy-induced nausea and vomiting
- beta 3-adrenoceptor agonists
- vanilloid receptors, such as capsaicin and resinferatoxin, which have some efficacy but have adverse events (AEs) such as urinary retention
- phosphodiesterase inhibitors
- gonadotropin-releasing–hormone antagonists
- vitamin D3–receptor analogs
- EP-1–receptor antagonists
- TRPV1–receptor antagonists
- antiepileptic drugs, such as gabapentin
VI. INTERSTITIAL CYSTITIS/PAINFUL BLADDER SYNDROME (IC/PBS)
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a debilitating chronic disease of unknown etiology characterized by urgency, frequency, and suprapubic pain at full bladder.27 Considered one of the chronic pelvic pain syndromes, the pathophysiology of IC/PBS is not well understood, and current treatment strategies remain suboptimal.54
Variations in disease definition and diagnostic criteria have hampered the accurate evaluation of the epidemiology of IC. The condition may affect 3.4 to 7.9 million women in the United States alone.55 Based on recent estimates, at least 197 of every 100,000 women and more than 41 of every 100,000 men in the United States are affected, but the prevalence may be even higher, affecting 1 in 4 or 5 women and 1 in 20 men.56 The majority of cases are mild-to-moderate in severity.57
IC is marked by urinary urgency, frequency, pelvic pain, or a combination of all of these without an obvious identifiable cause (such as infection or neoplastic process). Symptom flares can be associated with sexual relations, menstrual cycle, certain types of food, and stress.27
The pathogenesis of interstitial cystitis has been rather elusive. Currently, IC is thought to involve epithelial dysfunction and inflammatory events as well as peripheral and central nerve dysfunction. In such a model, an initiating event leads to dysfunctional urothelium, potassium leakage into tissue, and resulting afferent C fiber activation and release of substance P and other mediators. This leads to a cycle of mast cell activation and release of histamine and other mediators, which further exacerbates injury and epithelial leak.58
Diagnosis of IC should be made based on a thorough physical examination and the patient’s history of urgency/frequency and/or pain in the absence of bacterial infection. A symptom questionnaire can be used to establish the diagnosis.57 A potassium sensitivity test can also be considered.59 Urodynamic examinations can be performed in which patients receive cystoscopy with hydrodistention. Using this test, IC can be diagnosed if there are more than 10 glomerulations per quadrants in 3 or 4 quadrants of the bladder and if there is terminal hematuria.27 However, the importance of cystoscopy or urodynamics beyond symptom assessment is a matter of debate.57
Current treatments are usually unsuccessful in completely eradicating bladder pain and increasing bladder capacity. 60 Intravesical resiniferatoxin once was considered to be effective, but this has not been validated in a large-scale, multicenter trial.61 Besides hydrodistention (which has a short therapeutic duration), no long-term efficacy has been shown with any of the following: intravesical therapies, such as hyaluronic acid; Bacillus Calmette-Guérin; oral medications with pentosan polysulfate (PPS); cyclosporine A; or amitriptyline.62-65 The recent IC database study noted that loss of epithelial integrity is a predominant histopathological finding and may precede other histopathological findings in the bladder wall. The suburothelial space immediately below the basal lamina is well supplied with sensory nerves, which transmit the sensation of bladder fullness and response to bladder inflammation.66,67 Local inflammatory process might be induced through the afferent and efferent nerves in the suburothelial interstitial cellular network, which integrates the transmission of signals from the urothelium to the detrusor muscles in the bladder wall.68,69
In a rat chemical cystitis model, detrusor injection of onabotulinumtoxinA has been shown to have effects on increasing bladder capacity and compliance.70 In this regard, inhibition of neuroplasticity of the sensory fibers in the suburothelial space by intravesical onabotulinumtoxinA injections might have a good therapeutic effect targeting pain and sensory urgency in patients with IC/PBS.70-71
In another recent study, Kuo and Chancellor compared the therapeutic results of intravesical onabotulinumtoxinA 100 to 200 U plus hydrodistention vs hydrodistention alone.76
Sixty-seven patients with IC/PBS in whom conventional treatments had failed were enrolled. Forty-four patients received suburothelial injection with 200 U (n = 15) or 100 U (n = 29) of onabotulinumtoxinA followed by cystoscopic hydrodistention 2 weeks later (onabotulinumtoxinA group). The control group (23 patients) received the identical hydrodistention procedure without onabotulinumtoxinA injection. All patients were taking PPS and analgesics as baseline medication.76 The maximum bladder capacity during hydrodistention increased from 589 ± 182 mL to 714 ± 175 mL (P =.001), and from 646 ± 196 to 802 ± 228 mL (P<.001) 2 weeks after 200 U and 100 U onabotulinumtoxinA injections, respectively.76 The symptom score decreased in all three groups, but bladder pain visual analog scale reduction, increased functional bladder capacity, and increased cystometric bladder capacity were seen only in the onabotulinumtoxinA groups at 3 months. Among 44 patients in the onabotulinumtoxinA group, 31 (71%) had a successful result at 6 months , compared with only 8 (35%) patients treated with hydrodistention (P <.001).76 A successful result was reported in 24 (55%) and 13 (30%) patients in the onabotulinumtoxinA group at 12 and 24 months, respectively, but only in 6 (26%) and 4 (17%) controls (P =.002).76
Intravesical onabotulinumtoxinA injection plus hydrodistention may have dual effects: 1) inhibiting release of sensory nerve neurotransmitters, and 2) inhibiting acetylcholine release in the neuromuscular junctions of the detrusor. Hydrodistention alone may have an effect on increased bladder capacity and may excite the sensory nerves in suburothelium; together, these effects might potentiate the effect of onabotulinumtoxinA on sensory receptors in the bladder wall.
VII. NEUROTOXIN INJECTION TECHNIQUES FOR DSD, OAB AND INTERSTITIAL CYSTITIS
Several technical points should be made about injecting BoNT in the LUT.
Urethra
Urethral sphincter injection with onabotulinumtoxinA is performed by mixing 100 U to 200 U of onabotulinumtoxinA with approximately 4 mL of sterile saline just before injection. In the male patient, a rigid cystoscope and a standard cystoscopic injection needle are used to inject equal aliquots of onabotulinumtoxinA into the external sphincter at the 3-o’clock, 6-o’clock, 9-o’clock, and 12-o’clock positions. The preferred method in female patients is to use a fine-gauge spinal needle to inject onabotulinumtoxinA periurethrally at the 3-o’clock, 9-o’clock, and 12-o’clock positions approximately 1 cm from the urethral meatus. The needle is inserted parallel to the urethra to a depth of approximately 2 cm.
Alternatively, onabotulinumtoxinA can be injected transperineally to treat DSD. Tsai and colleagues examined injection of 100 U of onabotulinumtoxinA in the treatment of DSD in patients with spinal cord injury (SCI). The injection was made into the external sphincter using combined fluoroscopic and electromyographic guidance, using a Foley catheter for visualization of vesicourethral anatomy.76 Positive clinical outcomes were observed in all 18 patients—with mean reductions from baseline in PVR volume of 183 mL, leak-point pressure of 37 cm H2O, maximal intravesical pressure of 45 cm H2O, and maximal urethral pressure of 92 cm H2O. Chen and colleagues used transrectal ultrasound (TRUS) guidance to provide a transperineal injection of onabotulinumtoxinA to the external urethral sphincter in the treatment of DSD. However, while they found significant reductions in integrated electromyography (iEMG) and static and dynamic urethral pressure, there were no differences in detrusor pressure and detrusor leak-point pressure after treatment.77
Patients with symptoms of NDO, IDO, OAB, hypersensitive bladder, or IC/PBS refractory to conventional medical treatment are candidates for intravesical injections of BoNT. Injection of BoNT can paralyze and reduce the contractility of detrusor muscle, which explains its effectiveness as a treatment in patients with NDO or IDO.42,44 In addition, the immunoreactivity of purinergic P2X3 receptors and transient cationic (TRPV1) receptors on suburothelial sensory nerves was shown to be increased in patients with NDO and IDO and declined after detrusor injection of onabotulinumtoxinA.30,78
There is no universal consensus for the optimal dose or sites of BoNT injections in the treatment of refractory OAB or DO. Injection of 200 to 300 U of onabotulinumtoxinA is the most commonly used dose for NDO,30-33 100 to 200 U of onabotulinumtoxinA have been applied in treating IDO or OAB.34,50,51
a) Technical Points of Intravesical BoNT Injection
One important factor for a successful therapeutic outcome with onabotulinumtoxinA is adequate distribution of toxin into the suburothelial space and detrusor muscles. Desensitization of the mechanoreceptors on suburothelial sensory fibers can result in a decrease in the bladder urgency sensation and a reduction of sensory neuropeptide–mediated detrusor overactivity. Injection of onabotulinumtoxinA into detrusor muscles can cause paralysis of the affected muscle fibers.46 Together, these effects can decrease the bladder urgency sensation and increase bladder capacity. However, if the onabotulinumtoxinA is not adequately distributed into the bladder wall, or the toxin is injected outside the bladder wall, the desired effect may not be achieved.46 This might explain why some investigators used large doses of onabotulinumtoxinA in detrusor injections but the therapeutic effects were similar to those with suburothelial onabotulinumtoxinA injections.45,47 It is possible that much of the BoNT solution is injected too deep and outside the bladder wall with detrusor injections. To achieve a favorable therapeutic result, suburothelial injection of onabotulinumtoxinA seems to be a better route of injection than direct injection into the detrusor muscle.34
Although most of the onabotulinumtoxinA treatments for neurogenic detrusor overactivity (NDO) were performed by detrusor injection, there is no consensus as to the technique of onabotulinumtoxinA injection for IDO and OAB. Recent studies of onabotulinumtoxinA injection for OAB or IDO used suburothelial or intravesical injection techniques instead of detrusor injection.79 The technical point is targeting the suburothelial sensory pathway rather than paralysis of detrusor overactivity in treatment of OAB.66 However, because the bladder wall is thin, cystoscopy cannot differentiate the suburothelium from the detrusor layer. 66,76 Kuo compared the onabotulinumtoxinA effect on IDO among detrusor, suburothelial, and bladder base injections. They found no significant difference between detrusor and suburothelial injections.80 In a recent study using magnetic resonance imaging (MRI) in detecting distribution of BoNT-A throughout the bladder wall after injecting 300 U of onabotulinumtoxinA for NDO, about 17.6% of the BoNT-A solution was found outside the bladder dome, and the remaining BoNT-A could cover 25% to 33% of the bladder wall.81
The trigone and bladder base have been found to have abundant sensory fibers. Injections of onabotulinumtoxinA into these areas have been shown to have therapeutic effects on idiopathic urgency-frequency syndrome and IC/PBS.82 Although the trigone of the urinary bladder is rich in sensory fibers, the role of trigonal sensory fibers on bladder urgency sensation and detrusor overactivity has not been explored yet. 80 The sensation from the trigone might be related to bladder emptying rather than storage.80 Hence, treatment aimed at reducing sensation from the trigone might not improve the urgency sensation occurring during the bladder-filling phase.80Although vesicoureteral reflux might be a potential complication after onabotulinumtoxinA in these areas, there is no evidence of it so far.80,83 An advantage of trigonal injections of onabotulinumtoxinA is that detrusor underactivity does not develop after treatment. Another study that evaluated the effect of BoNT-A injections in the trigone on the antireflux mechanism confirmed the safety of trigone injections of onabotulinumtoxinA in relation to the development of vesicoureteral reflux or upper urinary tract damage.84
OnabotulinumtoxinA can be injected into the detrusor, suburothelium, or trigone to treat patients with OAB, IDO, or IC/PBS. One hundred units of onabotulinumtoxinA is usually reconstituted to 10 mL with normal saline. Detrusor injection can be performed by injecting onabotulinumtoxinA solution at 10 to 20 sites (Figure 3). The injection sites will be equally distributed with 0.5 to 1.0 mL for each injection. Suburothelial injection can be performed using a procedure identical to detrusor injections, except that the needle is inserted just into the suburothelial space and a ballooning formation is noted during infusion of onabotulinumtoxinA solution. The bladder volume is typically kept at 150 to 200 mL during the injection procedure. Patients usually report symptom improvement after a few days and continue to improve during the first month. 85 After this period, the patient will also feel a gradual increase of difficult urination and incomplete emptying.
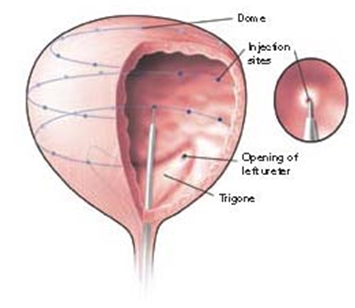
Figure 3. BoNT bladder injection technique. Adapted with permission from Chancellor.1
b) Safety of BoNT-A Injections
The main AEs of intravesical onabotulinumtoxinA injection are large increases in PVR volume and urinary tract infections (UTIs), which occur in about 20% to 43% of patients.45-48,80 UTIs are usually associated with the occurrence of large amounts of PVR.79 Other AEs, such as hematuria, micturition pain, and general weakness, are transient and easily overcome by conventional treatment. Resolution of these complications can be expected within the initial 2 weeks after the injection.46,86
Patients should be informed about what to expect after each treatment, particularly the side effects to look for. The nurse can be a key facilitator of such education and monitoring. For example, pain is rare but can last minutes to several hours after the procedure. However, this usually occurs only in patients with a history of bladder pain syndromes or in those with neuropathic pain. Hematuria usually does not continue past the procedure, and patients should be instructed to call the office if they pass any clots after the procedure. Anticoagulation therapy prior to and after the procedure is also a factor to consider. Patients should also be instructed to watch for any signs and symptoms of infection, which can develop days to weeks after the procedure. 87
The dose of onabotulinumtoxinA has a strong relationship to the occurrence of large PVR volume or urinary retention requiring clean intermittent catheterization (CIC). Kuo found that the rate of AEs increased with increasing doses of BoNT-A for IDO. A dose of 200 U of onabotulinumtoxinA yielded a 30% incidence of urinary retention, whereas none was found after injecting 100 U of onabotulinumtoxinA.34 A study conducted in a cohort of 34 patients showed that repeated injections of onabotulinumtoxinA at an initial dose of 200 U with subsequent injections between 100 U and 300 U are associated with significant improvement in OAB syndrome symptoms (frequency, urgency, and urgency incontinence) and QoL vs baseline in patients with IDO.88 The most common reported problems were difficulty emptying the bladder and urinary tract infection.89 Bladder base injection alone also has a lower incidence of urinary retention, but this treatment modality has a significantly shorter therapeutic period compared with bladder body injection. 89
In a randomized, controlled trial of onabotulinumtoxinA in women with OAB, Brubaker et al also found a 43% incidence of large PVR volume needing clean intermittent catheterization (CIC).89 Although large PVR volumes, UTIs, and chronic urinary retention remain obstacles for the wide application of onabotulinumtoxinA in the treatment of refractory detrusor overactivity (DO), there has been no factor to predict the occurrence of AEs after onabotulinumtoxinA injection. In a recent study, large PVR volumes requiring CIC occurred in 29% of patients treated with 200 U of onabotulinumtoxinA.90 Risk factors for incomplete emptying included lower maximum flow rate (Qmax), lower projected isovolumetric pressure, and lower bladder contractility index.90 Although, in another study, injecting 200 U of onabotulinumtoxinA has been found to be safe in elderly patients, the detrimental effect of retention on QoL can be considerable.91 However, a recent study by Kessler and colleagues conducted in women with IDO undergoing BoNT injections showed that change from baseline in Urogenital Distress Inventory and Incontinence Impact Questionnaire before and 4 weeks after BoNT were improved in women requiring CIC. In addition, these women did not experience an impaired QoL in the short term after onabotulinumtoxinA therapy.92 The authors suggest that all patients should be informed about the potential for CIC after onabotulinumtoxinA injections.
VIII. PROSTATE DISORDERS: BPH AND PROSTATITIS
Benign prostatic hyperplasia (BPH) and prostatitis are common genitourinary disorders that can cause lower urinary tract symptoms (LUTS). The symptoms of BPH and prostatitis overlap.93,94
BPH is an enlargement of the prostate gland from the progressive hyperplasia of stromal and glandular prostatic cells. BPH can lead to compression of the prostatic urethra that can be associated with voiding problems and LUTS.94,95
Prostatitis is a disorder of the prostate gland that is either inflammatory or non-inflammatory, leading to pelvic pain and voiding symptoms. Prostatitis can be either acute or chronic.93 This presentation will focus on chronic prostatitis, which typically has a substantial impact on pain, voiding, and sexual dysfunction, substantially reducing patients’ QoL.93,94
BPH is a major health concern for aging men. It is estimated that BPH affects 42% of men aged 51 to 60, 70% of men aged 61 to 70, and close to 90% of men aged 81 to 90.94,95 A recent survey found that men who develop prostatitis are more likely to develop BPH compared with men without a diagnosis of prostatitis.96 Other risk factors for BPH include age, functional testes, and family history of BPH.97
Prostatitis is the most common diagnosis for men under 50 years of age presenting in outpatient urology clinics, with a prevalence rate estimated between 2% and 9.7%.96,99,100One of the risk factors for prostatitis is a history of sexually transmitted disease.
BPH is associated with LUTS, increased prostate size, decreased urinary flow, and sometimes altered sexual function in the absence of other etiologies. BPH is a disorder that generally becomes more severe as the patient ages and the prostate grows, although symptoms do not always correlate with the size of the prostate. 95
Prostatitis can be associated with pelvic pain, voiding symptoms, and sexual dysfunction. Many men with prostatitis will not have objective findings of prostatic infection or inflammation and the condition may be asymptomatic. Baseline severity impacts the resolution with therapy—men with severe symptoms may suffer for years, while men who make an initial visit for a first episode tend to fare better than men with recurrent symptoms.96
BPH arises through a hyperplastic process that involves the fibromuscular stromal and glandular epithelial elements of the prostate, with a role for inflammation as well.98 The resulting increased size of the prostate compresses the prostatic urethra, leading to bladder outlet obstruction and LUTS.
Acute prostatitis is usually caused by a bacterial infection that occurs spontaneously or after manipulations of the urogenital tract.101Chronic bacterial prostatitis is often triggered by a complex bacterial infection, which can lead to the development of a biofilm in the prostatic acini.101 The etiology of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is not clear but may involve infection with organisms that are difficult to culture; functional obstruction and stimulation of pain nerve fibers; secretion of pro-inflammatory cytokines; and perhaps uroepithelial dysfunction.101
Since many men do not report LUTS symptoms, it is important to ask male patients over the age of 50 about urinary function in order to diagnose BPH. The clinician should take a medical history, using a questionnaire such as the International Prostate Symptom Score (IPSS), a physical examination including the digital rectal exam, urinalysis, as well as obtain prostate-specific antigen (PSA) testing where there is a concern about prostate cancer.95
Chronic bacterial prostatitis (CBP) can be diagnosed in patients presenting with pain, irritative symptoms during micturition, and sexual dysfunction. CBP can be demonstrated with the presence of certain levels and ratios of leukocytes, bacteria, and markers of inflammation in the urine, midstream urine, expressed prostatic secretion, or post-prostatic massage urine (per the 4-glass test or 2-glass test). For the diagnosis of CPPS, pain must be present for at least 3 months in the previous 6 months. The 4-glass and 2-glass tests as well as an ejaculatory test can be used to evaluate the inflammatory or noninflammatory form using markers such as peroxidase-positive leukocytes, elastase, and interleukin-8.101
BPH is typically managed through watchful waiting or medical management with alpha blockers, 5-alpha reductase inhibitors, anticholinergics, phosphodiesterase type 5 inhibitors, and combination approaches.102 More severe cases that lead to urine retention are typically managed surgically. Strategies include open prostatectomy, laser prostatectomy, transurethral resection of the prostate, transurethral incision of the prostate, and transurethral electrovaporization of the prostate. Less invasive surgical approaches include transurethral microwave therapy, transurethral needle ablation, and intraprostatic stents.95
CP/CPPS is generally treated with alpha-receptor blocker therapy for naïve newly diagnosed patients; antimicrobial therapy for antimicrobial naïve patients; and multimodal symptomatic therapy. Muscle relaxants may be used for functional abnormalities in the pelvic floor, although this is the area in which neurotoxins have a role. Nonsteroidal anti-inflammatories may be given for pain and anticholinergics for micturition problems.101
Role of Neurotoxins in Prostate Disorders
Doggweiler and colleagues studied chemical denervation by injection of onabotulinumtoxinA into the rat prostate and observed a generalized atrophy and apoptosis of glandular elements.103 Cholinergic innervation of the prostate gland has an important role in regulation of the functions of the prostate epithelium, with effects on growth and secretion, while noradrenergic innervation has been implicated in the contraction of smooth muscle and etiology of outflow obstruction accompanying BPH.104,105 OnabotulinumtoxinA, inhibiting the release of acetylcholine at the nerve terminal, can suppress the secretomotor function of acetylcholine on the prostate and result in a decrease in prostate weight. In rats as well as in dogs, onabotulinumtoxinA induces prostatic gland atrophy and apoptosis.103,106 In humans, increases in apoptotic activity at both epithelial and stromal components were noted after onabotulinumtoxinA injection, thus reducing the bulk or anatomical obstructive component of BPH in humans.107 In addition, Lin et al reported that injection of 200 U of onabotulinumtoxinA into the canine prostate significantly reduced the prostate urethral pressure response to intravenous norepinephrine and electrostimulation.108
g) Clinical Experience With BoNT for the Prostate
Available evidence suggests that intraprostatic BoNT injection improves lower urinary tract symptoms (LUTS) and flow rate but with minimal to modest decreases in prostate volume. The duration of effect appears to be 6 to 12 months.105 Urethral, perisphincteric, or intraprostatic BoNT-A injection might have therapeutic benefits in human nonbacterial prostatitis or chronic prostatic pain.105 Patients with BPH, chronic prostatitis, or chronic pelvic pain syndrome (CPPS) refractory to conventional treatments may have an alternative consideration with BoNT.105,109 In addition, older frail patients may benefit from this less invasive procedure. Silva and colleagues110 examined responses at both 6 months and 18 months after a single, intraprostatic injection of 200 U onabotulinumtoxinA in 21 men with refractory urinary retention secondary to BPH who were unfit for surgery. Mean prostatic volume decreased from 82 ± 16 mL at baseline to 49 ± 9.5 mL at month 6 but returned to baseline values at 18 months. The patients resumed spontaneous voiding at month 1. Qmax, PVR volumes, and International Prostate Symptom Score (IPSS) were maintained over the 18-month period.
An alternative form of BoNT-A, abobotulinumtoxinA, was studied as intraprostatic therapy in 72 elderly men with BPH. In this study, 300 to 600 U of abobotulinumtoxinA injected transperineally under TRUS guidance provided a significant decrease in IPSS and QoL score. PSA and prostate volume substantially dropped during the 6-month follow-up period but were not persistent at 12 months.111 Larger randomized trials are needed to clearly establish the mechanism by which BoNT affects the prostate as well as the ideal dose and duration of effect. 112
Technique of Prostate BoNT injection
Injections of BoNT into the prostate can be carried out transperineally, transrectally, or transurethrally. Of these three possible approaches, transperineal injection provides the lowest risk of UTI. However, transrectal prostatic injection is the procedure that urologists are most familiar with and most commonly use for prostate BoNT injection. Prostate injections are performed by mixing 100 to 200 U of onabotulinumtoxinA with saline just prior to injection. For those with a prostate volume larger than 60 mL, more than 200 U may be necessary. The preparation and positioning of the patient are identical to those used for transrectal or transperineal ultrasound-guided prostate biopsy. Some urologists may prefer transurethral prostate injection using a familiar cystoscope and injecting needle to approach the enlarged prostate gland.
During treatment, 200 U of onabotulinumtoxinA is reconstituted with 4 mL of normal saline. Chuang et al112 used a 23-gauge-long needle under TRUS guidance with the transverse and sagittal views to ensure proper placement of the needle as a bright spot in the center of each lateral lobe where 2 mL of BoNT are injected into each side (Figure 4). Under TRUS guidance, the injecting solution is adequately distributed within the volume of the prostate gland. Diffusion of hyperechoic BoNT over the lateral lobe of the prostate can be easily seen with TRUS monitoring. BoNT solution should be equally distributed to bilateral lobes, including the median lobe. After BoNT injection into the prostate, a certain percentage of patients might develop AEs, such as gross hematuria, difficult urination, perineal pain, or acute prostatitis. These AEs are caused by inadvertent penetration of the injection needle through the prostatic urethra in patients with asymmetry of the prostatic lobes, volume effect of the injected toxin, or inadequate sterility of the procedure. Selection of a small-gauge needle, careful insertion of the needle under sonographic guidance, small injecting volume, and adequate sterility usually can reduce these AEs to a minimum.
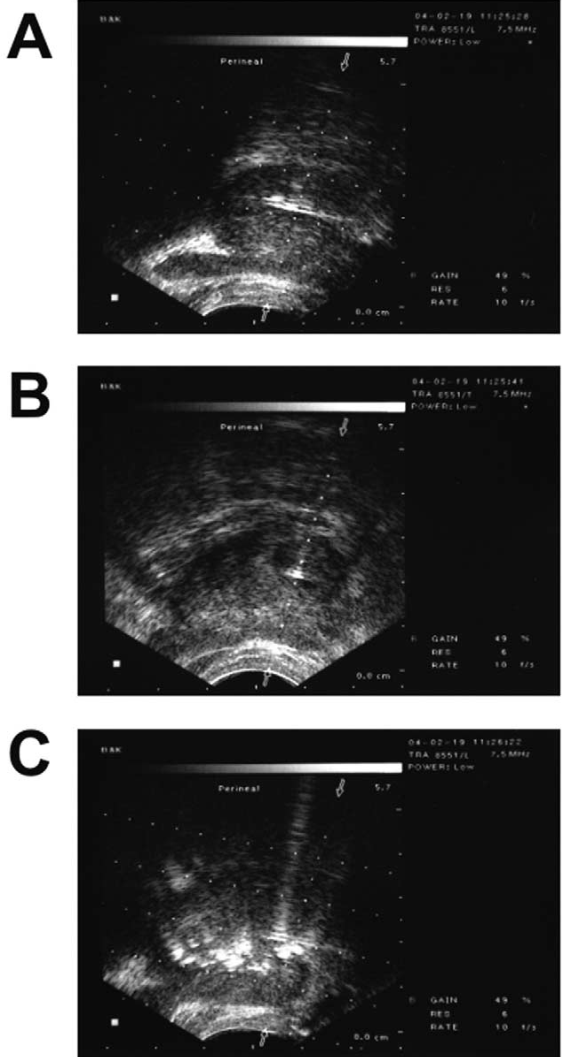
Figure 4. Clinical prostate BoNT injection technique. Confirmation of needle (black arrow) within the prostate: A, longitudinal view; B, transverse view. C, Diffusion of hyperechoic BoNT (black arrow) over the prostate immediately post-injection. Reprinted from Chuang et al.112 Copyright 2005, with permission from Elsevier
Several different investigational approaches are being studied to optimize the application of BoNT for LUTS. For example, intraprostatic onabotulinumtoxinA appears to have benefit in a rat model of prostatitis.113 In addition, there are alternative BoNT formulations being studied, including onabotulinumtoxinA delivered using liposomes to increase targeting to the submucosal nerve plexus.114 The feasibility of direct intravesicular instillation of onabotulinumtoxinA (300 U with 50% demethyl sulfoxide [DMSO]) has been shown in a phase 1-2 study in patients with IDO.115 Further, onabotulinumtoxinA has been studied in combination with endoscopic treatment of vesicoureteral reflux caused by neurogenic bladder in children.116
BoNT has been shown to be effective for wide variety of lower urinary tract symptoms that involve muscular hypercontractility, hypersensitivity, and glandular hypertrophy. Advances have been made in our understanding of how BoNT works, as well as the agent’s clinical application and results. Currently, the optimal treatment paradigms (ie, dose, dilution, number and location of injections) for each disorder remain to be established. In addition, the development of a simpler and more effective method for BoNT delivery into the bladder without the need for injection and with lower risk of urinary retention may be a new advancement. BoNT is currently undergoing clinical trials seeking regulatory approval for neurogenic detrusor overactivity, idiopathic detrusor overactivity, and benign prostatic hyperplasia indications. Teasing out the “right” dose that will maximize efficacy and minimize the side effect of urinary retention is a key goal for the future.1
References
1. Chancellor MB. Ten years single surgeon experience with botulinum toxin in the urinary tract; clinical observations and research discovery. Int Urol Nephrol 2010;42(2): 383-91.
2. Llorente C. New concepts in epidemiology of lower urinary tract symptoms in men. Eur Urol Suppl 2010;9: 477-81.
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187(1): 116-26.
4. Chancellor M, Yoshimura N. Physiology and pharmacology of the bladder and urethra. In: Walsh P, Retik A, Vaughan E, Wein A, editors. Campbell’s Urology. Philadelphia, PA: WB Saunders, 2002:831-86.
5. Erol B, Kocak T, Kadioglu A, et al. [The relationship between level of injury and bladder behavior in patients with post-traumatic spinal cord injury]. Ulus Travma Acil Cerrahi Derg 2009;15(4): 377-82.
6. Meng NH, Lo SF, Chou LW, Yang PY, Chang CH, Chou EC. Incomplete bladder emptying in patients with stroke: is detrusor external sphincter dyssynergia a potential cause? Arch Phys Med Rehabil 2010;91(7): 1105-9.
7. Onal B, Siva A, Buldu I, Demirkesen O, Cetinel B. Voiding dysfunction due to multiple sclerosis: a large scale retrospective analysis. Int Braz J Urol 2009;35(3): 326-33.
8. Jost WH, Naumann M. Botulinum toxin in neuro-urological disorders. Mov Disord 2004;19 Suppl 8: S142-5.
9. De EJ, Patel CY, Tharian B, Westney OL, Graves DE, Hairston JC. Diagnostic discordance of electromyography (EMG) versus voiding cystourethrogram (VCUG) for detrusor-external sphincter dyssynergy (DESD). Neurourol Urodyn 2005;24(7): 616-21.
10. Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139(5): 919-22.
11. Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164(3 Pt 1): 692-7.
12. Smith CP, Somogyi GT, Chancellor MB. Botulinum toxin: poisoning the spastic bladder and urethra. Rev Urol 2002;4(2): 61-8.
13. Smith CP, Somogyi GT, Chancellor MB. Emerging role of botulinum toxin in the treatment of neurogenic and non-neurogenic voiding dysfunction. Curr Urol Rep 2002;3(5): 382-7.
14. Dykstra DD, Sidi AA. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: a double-blind study. Arch Phys Med Rehabil 1990;71(1): 24-6.
15. Schurch B. The role of botulinum toxin in neurourology. Drugs Today (Barc) 2004;40(3): 205-12.
16. Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB. Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 1996;155(3): 1023-9.
17. de Seze M, Petit H, Gallien P, et al. Botulinum A toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002;42(1): 56-62.
18. Kuo HC. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J Urol 2003;170(5): 1908-12.
19. Smith CP, Nishiguchi J, O'Leary M, Yoshimura N, Chancellor MB. Single-institution experience in 110 patients with botulinum toxin A injection into bladder or urethra. Urology 2005;65(1): 37-41.
20. Dressler D, Eleopra R. Clinical use of non-A botulinum toxins: botulinum toxin type B. Neurotox Res 2006;9(2-3): 121-5.
21. Yoshida M, Masunaga K, Nagata T, Yono M, Homma Y. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: pathophysiology and pharmacotherapy of overactive bladder. J Pharmacol Sci 2010;112(2): 128-34.
22. Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 2001;87(9): 760-6.
23. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20(6): 327-36.
24. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50(6): 1306-14; discussion 14-5.
25. Homma Y, Kakizaki H, Gotoh M, et al. [Epidemiologic survey on lower urinary tract symptoms in Japan.] (in Japanese). J Neurogenic Bladder Soc 2003;14: 266-77.
26. Morris AR, Westbrook JI, Moore KH. A longitudinal study over 5 to 10 years of clinical outcomes in women with idiopathic detrusor overactivity. BJOG 2008;115(2): 239-46.
27. Chung MK, Butrick CW, Chung CW. The overlap of interstitial cystitis/painful bladder syndrome and overactive bladder. JSLS 2010;14(1): 83-90.
28. Agency for Health Care Policy and Research (AHCPR). Urinary incontinence in adults: clinical practice guideline updates. Agency for Health Care Policy and Research, Rockville, MD, March 1996. http://www.ahrq.gov/clinic/uiovervw.htm . Accessed August 31, 2010.
29. Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 2008;54(3): 543-62.
30. Giannantoni A, Di Stasi SM, Nardicchi V, et al. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol 2006;175(6): 2341-4.
31. Schulte-Baukloh H, Schobert J, Stolze T, Sturzebecher B, Weiss C, Knispel HH. Efficacy of botulinum-A toxin bladder injections for the treatment of neurogenic detrusor overactivity in multiple sclerosis patients: an objective and subjective analysis. Neurourol Urodyn 2006;25(2): 110-5.
32. Reitz A, Stohrer M, Kramer G, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol 2004;45(4): 510-5.
33. Grosse J, Kramer G, Stohrer M. Success of repeat detrusor injections of botulinum a toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur Urol 2005;47(5): 653-9.
34. Kuo HC. Therapeutic effects of suburothelial injection of botulinum a toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006;67(2): 232-6.
35. Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron RL. Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur Urol 2007;52(3): 850-8.
36. Herschorn S, Gajewski J, Ethans K, et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence – a randomized double-blind trial (abstr 33). Presented at International Continence Society, September, 2009, San Francisco, CA 2009.
37. Ghalayini IF, Al-Ghazo MA, Elnasser ZA. Is efficacy of repeated intradetrusor botulinum toxin type A (Dysport) injections dose dependent? Clinical and urodynamic results after four injections in patients with drug-resistant neurogenic detrusor overactivity. Int Urol Nephrol 2009;41(4): 805-13.
38. Kajbafzadeh AM, Nikfarjam L, Mahboubi AH, Dianat S. Antibody formation following botulinum toxin type A (Dysport) injection in children with intractable bladder hyper-reflexia. Urology 2010;76(1): 233-7.
39. Dykstra D, Enriquez A, Valley M. Treatment of overactive bladder with botulinum toxin type B: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct 2003;14(6): 424-6.
40. Ghei M, Maraj BH, Miller R, et al. Effects of botulinum toxin B on refractory detrusor overactivity: a randomized, double-blind, placebo controlled, crossover trial. J Urol 2005;174(5): 1873-7; discussion 77.
41. Hirst GR, Watkins AJ, Guerrero K, et al. Botulinum toxin B is not an effective treatment of refractory overactive bladder. Urology 2007;69(1): 69-73.
42. Kessler TM, Danuser H, Schumacher M, Studer UE, Burkhard FC. Botulinum A toxin injections into the detrusor: an effective treatment in idiopathic and neurogenic detrusor overactivity? Neurourol Urodyn 2005;24(3): 231-6.
43. Rajkumar GN, Small DR, Mustafa AW, Conn G. A prospective study to evaluate the safety, tolerability, efficacy and durability of response of intravesical injection of botulinum toxin type A into detrusor muscle in patients with refractory idiopathic detrusor overactivity. BJU Int 2005;96(6): 848-52.
44. Popat R, Apostolidis A, Kalsi V, Gonzales G, Fowler CJ, Dasgupta P. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol 2005;174(3): 984-9.
45. Schulte-Baukloh H, Weiss C, Stolze T, Sturzebecher B, Knispel HH. Botulinum-A toxin for treatment of overactive bladder without detrusor overactivity: urodynamic outcome and patient satisfaction. Urology 2005;66(1): 82-7.
46. Kuo HC. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004;63(5): 868-72.
47. Anger JT, Weinberg A, Suttorp MJ, Litwin MS, Shekelle PG. Outcomes of intravesical botulinum toxin for idiopathic overactive bladder symptoms: a systematic review of the literature. J Urol 2010;183(6): 2258-64.
48. Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010;184(6): 2416-22.
49. Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 2009;181(6): 2608-15.
50. Werner M, Schmid DM, Schussler B. Efficacy of botulinum-A toxin in the treatment of detrusor overactivity incontinence: a prospective nonrandomized study. Am J Obstet Gynecol 2005;192(5): 1735-40.
51. Schmid DM, Sauermann P, Werner M, et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholinergics. J Urol 2006;176(1): 177-85.
52. Cohen BL, Barboglio P, Rodriguez D, Gousse AE. Preliminary results of a dose-finding study for botulinum toxin-A in patients with idiopathic overactive bladder: 100 versus 150 units. Neurourol Urodyn 2009;28(3): 205-8.
53. Watanabe JH, Campbell JD, Ravelo A, Chancellor MB, Kowalski J, Sullivan SD. Cost analysis of interventions for antimuscarinic refractory patients with overactive bladder. Urology 2010;76(4): 835-40.
54. Nickel JC. A new approach to understanding and managing chronic prostatitis and interstitial cystitis. Rev Urol 2010;12(1): 67-68.
55. Clemens J, Stoto M, Elliott M, et al. Prevalence of interstitial cystitis/painful bladder syndrome in the United States (abstr 261). Presented at International Continence Society 39th Annual Meeting; September 29-October 3, 2009; San Francisco, CA.
56. Parsons JK, Kurth K, Sant GR. Epidemiologic issues in interstitial cystitis. Urology 2007;69(4 Suppl): 5-8.
57. Evans RJ, Sant GR. Current diagnosis of interstitial cystitis: an evolving paradigm. Urology 2007;69(4 Suppl): 64-72.
58. Moldwin RM, Evans RJ, Stanford EJ, Rosenberg MT. Rational approaches to the treatment of patients with interstitial cystitis. Urology 2007;69(4 Suppl): 73-81.
59. Parsons CL, Zupkas P, Parsons JK. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology 2001;57(3): 428-32; discussion 32-3.
60. Hanno PM, Sant GR. Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association scientific conference on interstitial cystitis. Urology 2001;57(6 Suppl 1): 2-6.
61. Payne CK, Mosbaugh PG, Forrest JB, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol 2005;173(5): 1590-4.
62. Nickel JC, Barkin J, Forrest J, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology 2005;65(4): 654-8.
63. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol 2003;170(3): 810-5.
64. Hanno PM, Buehler J, Wein AJ. Use of amitriptyline in the treatment of interstitial cystitis. J Urol 1989;141(4): 846-8.
65. Sairanen J, Forsell T, Ruutu M. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol 2004;171(6 Pt 1): 2138-41.
66. Brady CM, Apostolidis AN, Harper M, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int 2004;93(6): 770-6.
67. Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000;407(6807): 1011-5.
68. Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol 1999;277(2 Pt 1): C271-9.
69. Vera PL, Wang X, Meyer-Siegler KL. Neural control of substance P induced up-regulation and release of macrophage migration inhibitory factor in the rat bladder. J Urol 2008;180(1): 373-8.
70. Cayan S, Coskun B, Bozlu M, Acar D, Akbay E, Ulusoy E. Botulinum toxin type A may improve bladder function in a rat chemical cystitis model. Urol Res 2003;30(6): 399-404.
71. Steers WD, Tuttle JB. Mechanisms of Disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 2006;3(2): 101-10.
72. Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology 2004;64(5): 871-5; discussion 75.
73. Kuo HC. Preliminary results of suburothelial injection of botulinum a toxin in the treatment of chronic interstitial cystitis. Urol Int 2005;75(2): 170-4.
74. Giannantoni A, Costantini E, Di Stasi SM, Tascini MC, Bini V, Porena M. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: a pilot study. Eur Urol 2006;49(4): 704-9.
75. Giannantoni A, Porena M, Costantini E, Zucchi A, Mearini L, Mearini E. Botulinum A toxin intravesical injection in patients with painful bladder syndrome: 1-year followup. J Urol 2008;179(3): 1031-4.
76. Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009;104(5): 657-61.
77. Tsai SJ, Ying TH, Huang YH, Cheng JW, Bih LI, Lew HL. Transperineal injection of botulinum toxin A for treatment of detrusor sphincter dyssynergia: localization with combined fluoroscopic and electromyographic guidance. Arch Phys Med Rehabil 2009;90(5): 832-6.
78. Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 2005;174(3): 977-82; discussion 82-3.
79. Stern JA, Hsieh YC, Schaeffer AJ. Residual urine in an elderly female population: novel implications for oral estrogen replacement and impact on recurrent urinary tract infection. J Urol 2004;171(2 Pt 1): 768-70.
80. Kuo HC. Comparison of effectiveness of detrusor, suburothelial and bladder base injections of botulinum toxin a for idiopathic detrusor overactivity. J Urol 2007;178(4 Pt 1): 1359-63.
81. Mehnert U, Boy S, Schmid M, et al. A morphological evaluation of botulinum neurotoxin A injections into the detrusor muscle using magnetic resonance imaging. World J Urol 2009;27(3): 397-403.
82. Zermann D, Ishigooka M, Schubert J, Schmidt R. Trigonum and bladder base injection of botulinumtoxin A (BTX) in patients with severe urgency-frequency-syndrome refractory to conservative medical treatment and electrical stimulation. Neurourol Urodynam 2001;20: 412-13.
83. Karsenty G, Elzayat E, Delapparent T, St-Denis B, Lemieux MC, Corcos J. Botulinum toxin type a injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J Urol 2007;177(3): 1011-4.
84. Mascarenhas F, Cocuzza M, Gomes CM, Leao N. Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn 2008;27(4): 311-4.
85. Kalsi V, Apostolidis A, Gonzales G, Elneil S, Dasgupta P, Fowler CJ. Early effect on the overactive bladder symptoms following botulinum neurotoxin type A injections for detrusor overactivity. Eur Urol 2008;54(1): 181-7.
86. Kuo HC. Clinical effects of suburothelial injection of botulinum A toxin on patients with nonneurogenic detrusor overactivity refractory to anticholinergics. Urology 2005;66(1): 94-8.
87. O’Leary M, Dierich M. Urinary tract dysfunction in neurological disorders: the nurses’ role in assessment and management. J Neuroscience Nursing 2010;42: E8-E23.
88. Sahai A, Dowson C, Khan MS, Dasgupta P. Repeated injections of botulinum toxin-A for idiopathic detrusor overactivity. Urology 2010;75(3): 552-8.
89. Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008;180(1): 217-22.
90. Sahai A, Sangster P, Kalsi V, Khan MS, Fowler CJ, Dasgupta P. Assessment of urodynamic and detrusor contractility variables in patients with overactive bladder syndrome treated with botulinum toxin-A: is incomplete bladder emptying predictable? BJU Int 2009;103(5): 630-4.
91. White WM, Pickens RB, Doggweiler R, Klein FA. Short-term efficacy of botulinum toxin a for refractory overactive bladder in the elderly population. J Urol 2008;180(6): 2522-6.
92. Kessler TM, Khan S, Panicker J, Roosen A, Elneil S, Fowler CJ. Clean intermittent self-catheterization after botulinum neurotoxin type A injections: short-term effect on quality of life. Obstet Gynecol 2009;113(5): 1046-51.
93. Krieger JN, Nyberg L, Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA 1999;282(3): 236-7.
94. Nickel JC. The overlapping lower urinary tract symptoms of benign prostatic hyperplasia and prostatitis. Curr Opin Urol 2006;16(1): 5-10.
95. Tanguay S, Awde M, Brock G, et al. Diagnosis and management of benign prostatic hyperplasia in primary care. Can Urol Assoc J 2009;3(3 Suppl 2): S92-S100.
96. St Sauver JL, Jacobson DJ, McGree ME, Girman CJ, Lieber MM, Jacobsen SJ. Longitudinal association between prostatitis and development of benign prostatic hyperplasia. Urology 2008;71(3): 475-9; discussion 79.
97. Tang J, Yang J. Etiopathogenesis of benign prostatic hypeprlasia. Indian J Urol 2009;25(3): 312-7.
98. Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents 2008;31 Suppl 1: S85-90.
99. Collins MM, Stafford RS, O'Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol 1998;159(4): 1224-8.
100. Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology 1998;51(4): 578-84.
101. Wagenlehner FM, Naber KG, Bschleipfer T, Brahler E, Weidner W. Prostatitis and male pelvic pain syndrome: diagnosis and treatment. Dtsch Arztebl Int 2009;106(11): 175-83.
102. Laborde EE, McVary KT. Medical management of lower urinary tract symptoms. Rev Urol 2009;11(Suppl 1): S19-25.
103. Doggweiler R, Zermann DH, Ishigooka M, Schmidt RA. Botox-induced prostatic involution. Prostate 1998;37(1): 44-50.
104. Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol 2000;20(4): 193-206.
105. Chuang Y, Chancellor M. The application of botulinum toxin in the prostate. J Urol 2006;176: 2376-86.
106. Chuang YC, Huang CC, Kang HY, et al. Novel action of botulinum toxin on the stromal and epithelial components of the prostate gland. J Urol 2006;175(3 Pt 1): 1158-63.
107. Chuang YC, Tu CH, Huang CC, et al. Intraprostatic injection of botulinum toxin type-A relieves bladder outlet obstruction in human and induces prostate apoptosis in dogs. BMC Urol 2006;6: 12.
108. Lin AT, Yang AH, Chen KK. Effects of botulinum toxin A on the contractile function of dog prostate. Eur Urol 2007;52(2): 582-9.
109. Kuo HC. Prostate botulinum A toxin injection--an alternative treatment for benign prostatic obstruction in poor surgical candidates. Urology 2005;65(4): 670-4.
110. Silva J, Pinto R, Carvalho T, et al. Intraprostatic Botulinum Toxin Type A injection in patients with benign prostatic enlargement: duration of the effect of a single treatment. BMC Urol 2009;9: 9.
111. Nikoobakht M, Daneshpajooh A, Ahmadi H, et al. Intraprostatic botulinum toxin type A injection for the treatment of benign prostatic hyperplasia: Initial experience with Dysport. Scand J Urol Nephrol 2010;44(3): 151-7.
112. Chuang Y, Chiang P, Huang C, Yoshimura N, Chancellor M. Botulinum toxin type A improves benign prostatic hyperplasia symptoms in patients with small prostates. Urology 2005;66: 775-79.
113. Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB. Intraprostatic botulinum toxin a injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J Urol 2008;180(2): 742-8.
114. Chuang YC, Tyagi P, Huang CC, et al. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol 2009;182(2): 786-92.
115. Petrou SP, Parker AS, Crook JE, Rogers A, Metz-Kudashick D, Thiel DD. Botulinum a toxin/dimethyl sulfoxide bladder instillations for women with refractory idiopathic detrusor overactivity: a phase 1/2 study. Mayo Clin Proc 2009;84(8): 702-6.
116. Neel KF, Salem M, Soliman S. Total endoscopic management (TEM approach) of children with non-compliant neuropathic bladder: a preliminary report. J Pediatr Urol 2008;4(2): 124-6.






